Introduction
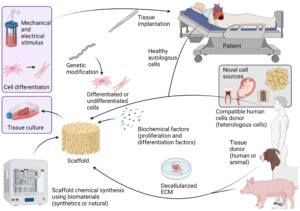
Tissue engineering represents a revolutionary approach to medicine, aiming to regenerate, repair, or replace damaged tissues and organs. At the heart of this burgeoning field lies the creation of scaffolds, which serve as the structural frameworks upon which cells can grow and develop into functional tissues. Polymers, with their versatility and tunability, have emerged as essential materials for scaffold fabrication. This post will delve into the intricate process of creating tissue engineering scaffolds using polymers, offering insights into the various methods, materials, and considerations involved.
1. The Importance of Scaffolds in Tissue Engineering
In tissue engineering, scaffolds provide the necessary support structure for cell attachment, proliferation, and differentiation. They mimic the extracellular matrix (ECM), offering a conducive environment for tissue formation. The choice of material and design of the scaffold directly impacts its efficacy. Polymers are favored due to their ability to be engineered to meet specific mechanical and biological requirements.
1.1. Functions of Scaffolds
- Structural Support: Scaffolds maintain the shape and integrity of the growing tissue.
- Cell Interaction: They facilitate cell adhesion, migration, and proliferation.
- Nutrient Transport: Scaffolds allow for the diffusion of nutrients and removal of waste products.
2. Types of Polymers Used in Scaffold Fabrication
Polymers used in tissue engineering can be broadly classified into natural and synthetic categories. Each type offers distinct advantages and challenges.
2.1. Natural Polymers
Natural polymers, such as collagen, gelatin, and chitosan, are biocompatible and often mimic the natural ECM. They support cell attachment and proliferation but can be limited by variability and lower mechanical strength.
- Collagen: Widely used due to its abundance in the human body and excellent biocompatibility.
- Gelatin: A derivative of collagen, gelatin is biodegradable and supports cell adhesion.
- Chitosan: Derived from chitin, chitosan is biodegradable and has antimicrobial properties.
2.2. Synthetic Polymers
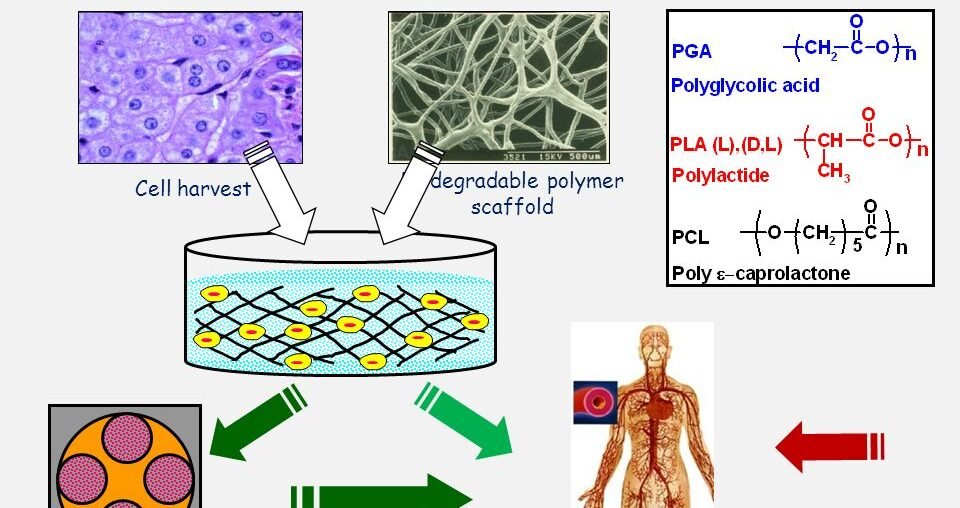
Synthetic polymers, such as polylactic acid (PLA), polyglycolic acid (PGA), and polycaprolactone (PCL), offer greater control over mechanical properties and degradation rates. However, they may lack the bioactivity of natural polymers.
- Polylactic Acid (PLA): Biodegradable and customizable, PLA is commonly used for its strength and biocompatibility.
- Polyglycolic Acid (PGA): Known for its rapid degradation, PGA is often used in combination with other polymers.
- Polycaprolactone (PCL): Notable for its long degradation time and excellent mechanical properties.
3. Methods for Creating Polymer Scaffolds
The fabrication of polymer scaffolds involves various techniques, each with its advantages and limitations. Key methods include solvent casting, electrospinning, and 3D printing.
3.1. Solvent Casting and Particulate Leaching
This method involves dissolving the polymer in a solvent, casting it into a mold, and incorporating porogens to create a porous structure. The porogens are then leached out, leaving behind a scaffold with interconnected pores.
3.2. Electrospinning
Electrospinning creates nanofibrous scaffolds that closely mimic the ECM. A polymer solution is subjected to a high voltage, creating fine fibers that are collected on a grounded target. This method is prized for its ability to produce highly porous and bioactive scaffolds.
3.3. 3D Printing
3D printing, or additive manufacturing, allows for precise control over scaffold architecture. Polymers are printed layer by layer, enabling the creation of complex and patient-specific structures. This technique is highly versatile and can incorporate multiple materials within a single scaffold.
4. Design Considerations for Polymer Scaffolds
Designing an effective tissue engineering scaffold requires careful consideration of various factors, including porosity, mechanical properties, and biodegradability.
4.1. Porosity
Porosity is crucial for nutrient transport and cell migration. An ideal scaffold should have interconnected pores of appropriate size to facilitate these processes. The porosity must be balanced with the mechanical strength to ensure scaffold stability.
4.2. Mechanical Properties
The mechanical properties of the scaffold must match the tissue it aims to replace. For instance, bone scaffolds require high stiffness and strength, while soft tissue scaffolds should be more flexible.
4.3. Biodegradability
The scaffold should degrade at a rate that matches tissue formation, providing support until the new tissue can sustain itself. The degradation products should be non-toxic and easily eliminated by the body.
Conclusion
Creating tissue engineering scaffolds with polymers is a complex yet rewarding endeavor that holds immense potential for regenerative medicine. By understanding the types of polymers, fabrication methods, and design considerations, researchers and practitioners can develop effective scaffolds tailored to specific medical applications. The future of tissue engineering is bright, with continued advancements in polymer technology paving the way for innovative therapies and improved patient outcomes.
We invite readers to share their thoughts, questions, and experiences in the comments below. Your feedback is invaluable as we continue to explore the fascinating world of tissue engineering and polymers.




1 Comment
Pingback: How to Utilize Polymers for Tissue Engineering Applications - greenpolymershub.com